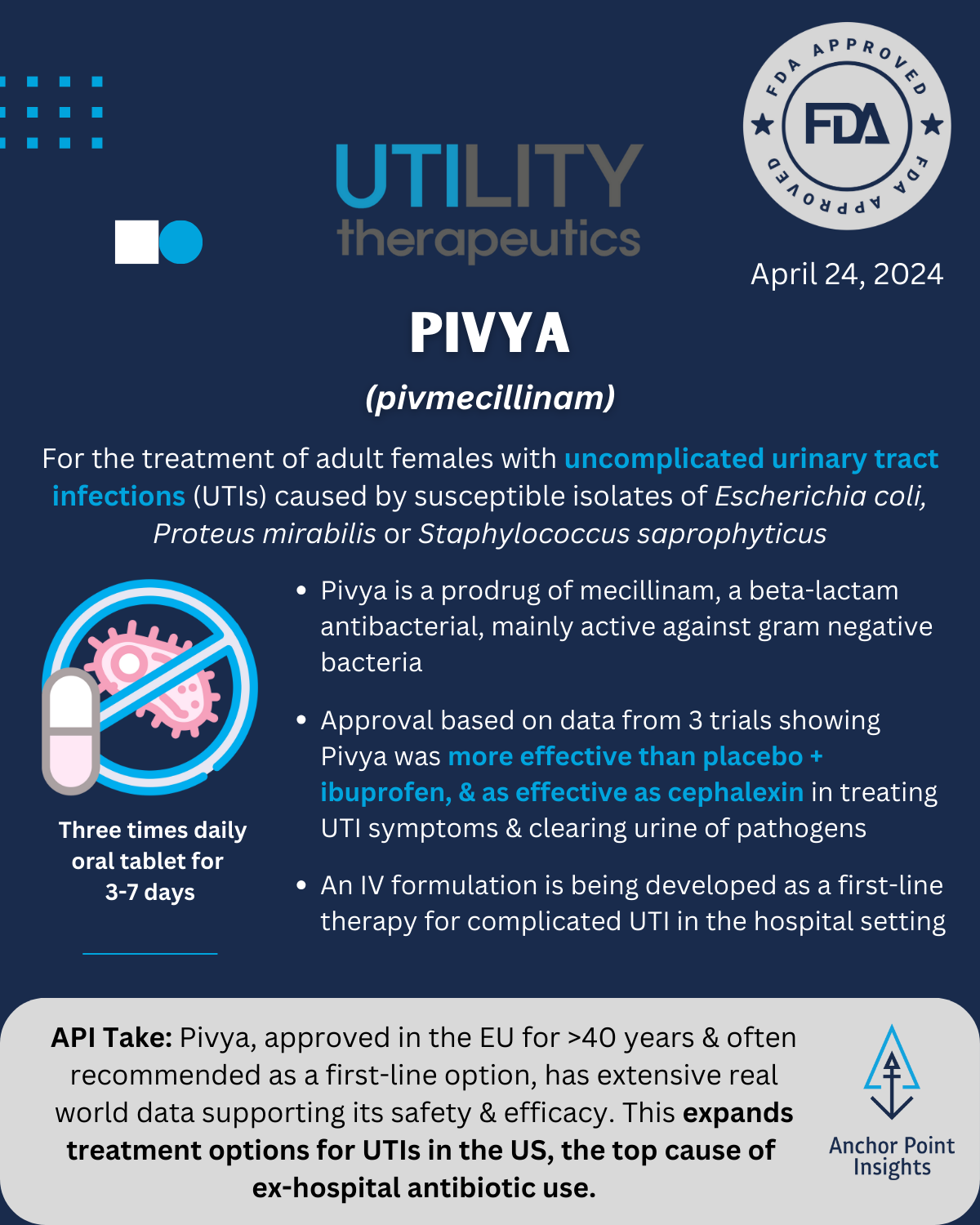
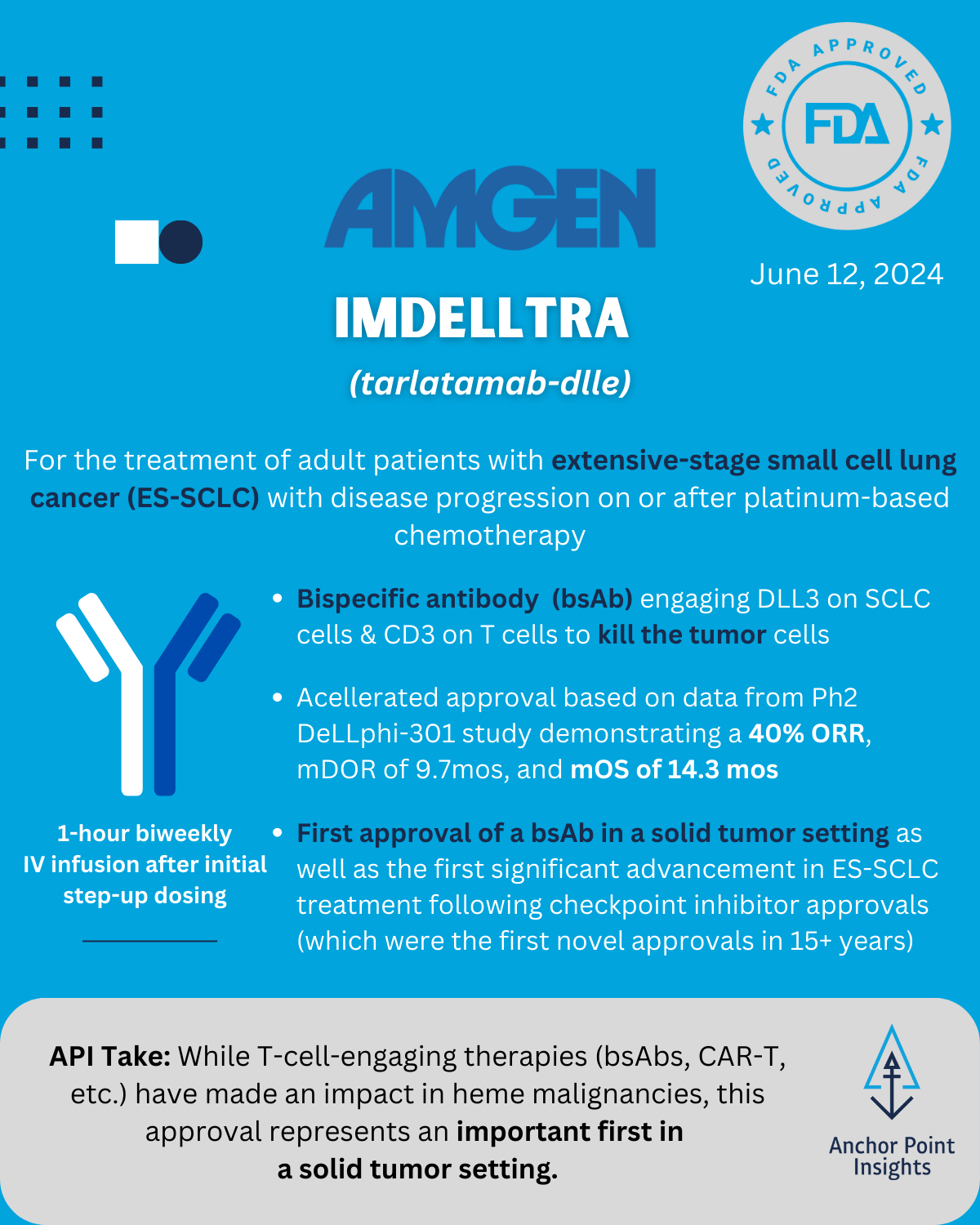
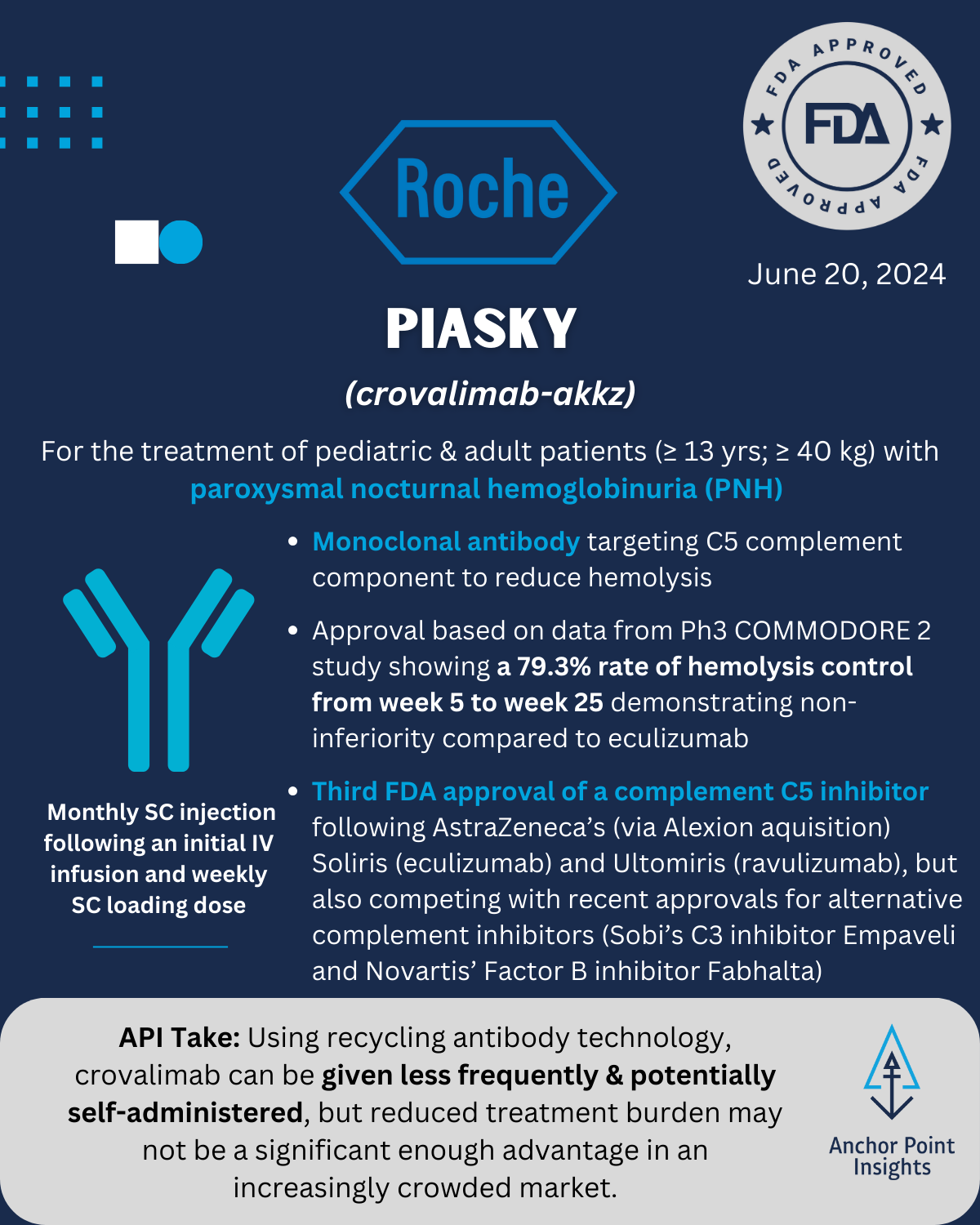
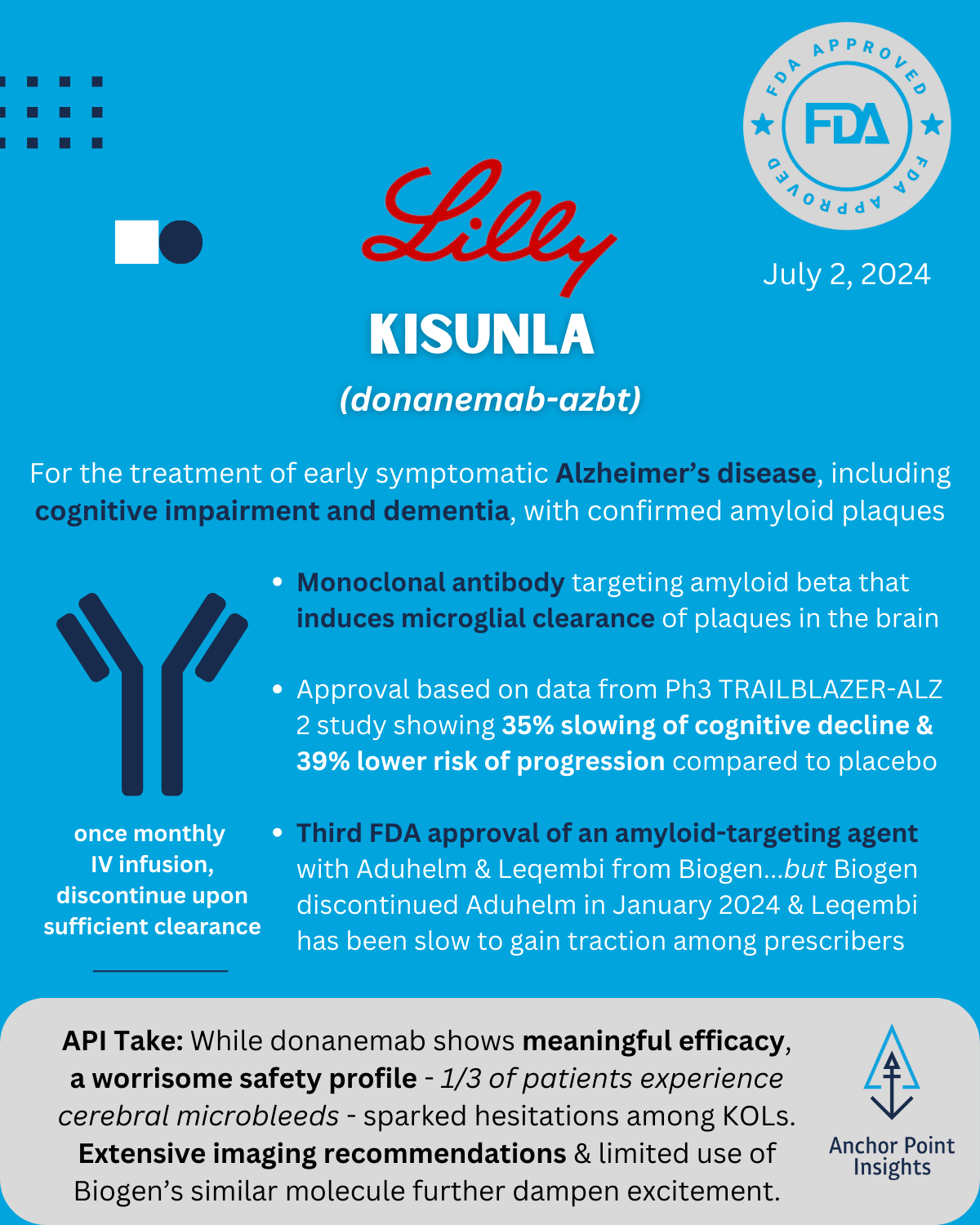
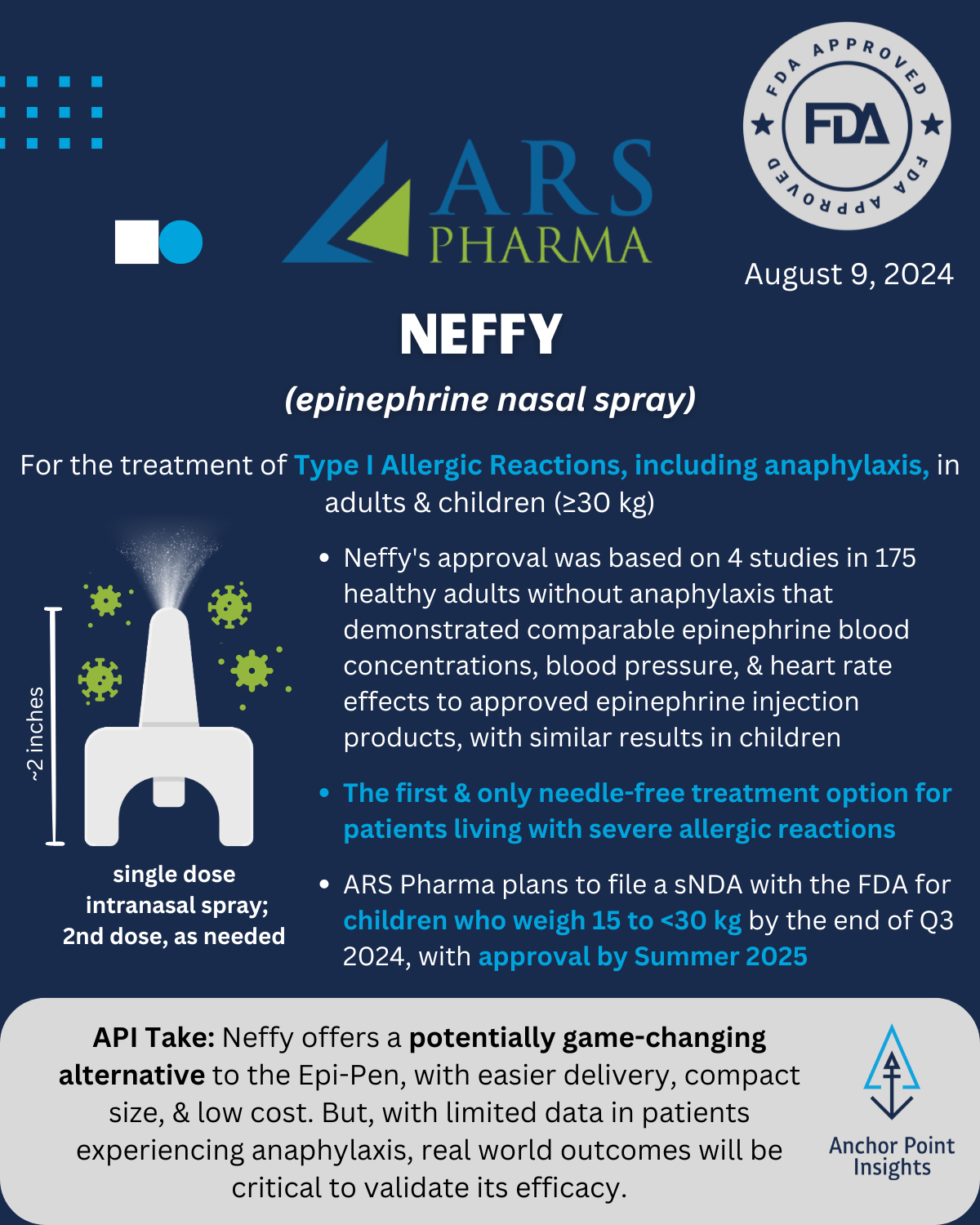
The FDA has had a busy year with 29 novel approvals to date. The API team has weighed in on a few of this summer’s approvals, highlighting 5 of the drugs we anticipate having major implications to existing therapeutic landscapes — or not. Read on for our take on Utility Therapeutics’ Pivya for UTIs, Amgen’s Imdelltra for Small Cell Lung Cancer, Roche’s Piasky for PNH, Lilly’s Kisunla for Alzheimer’s, and ARS Pharma’s Neffy for Type I Allergic Reactions.