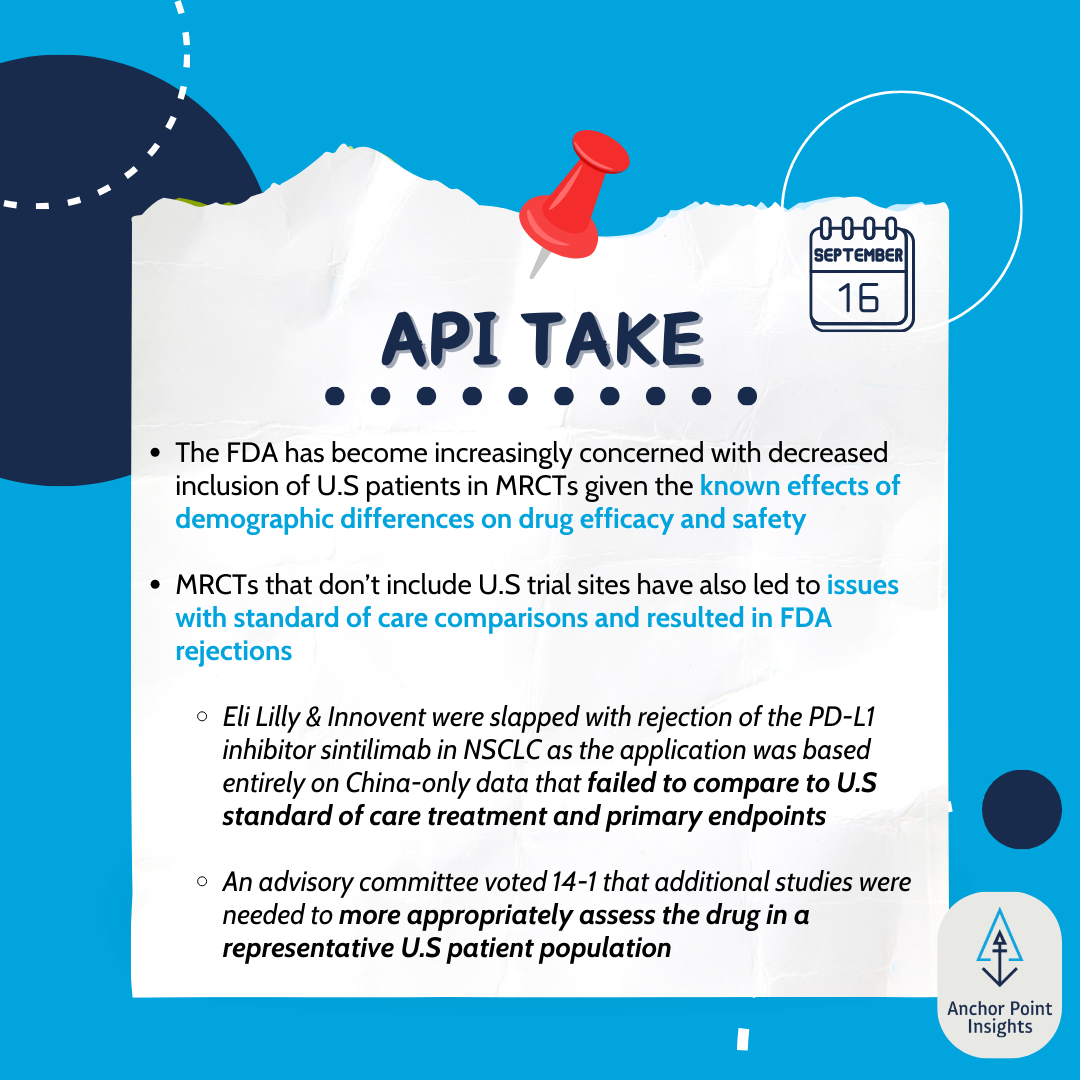
The FDA has issued draft guidance concerning multiregional clinical trials conducted for oncology indications. This is a significant step forward in light of decreased inclusion of US patients in such trials. These recommendations will help ensure the quality of global clinical trial data and its applicability to US patient populations.
Source: https://lnkd.in/gT__m9Ng